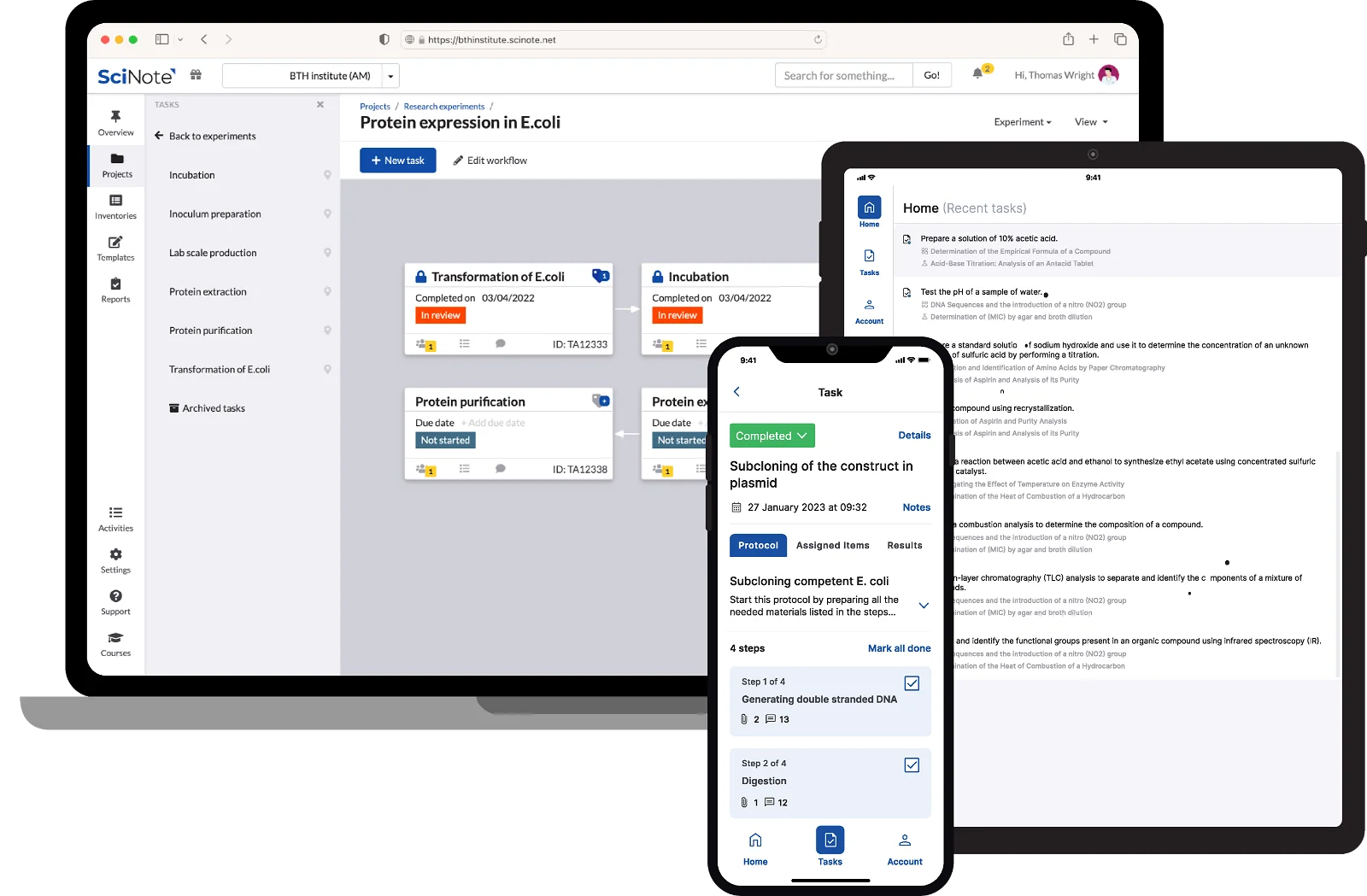
A Single Platform for Sample and Data Management – Top Tier Service for your Clinical and Research data
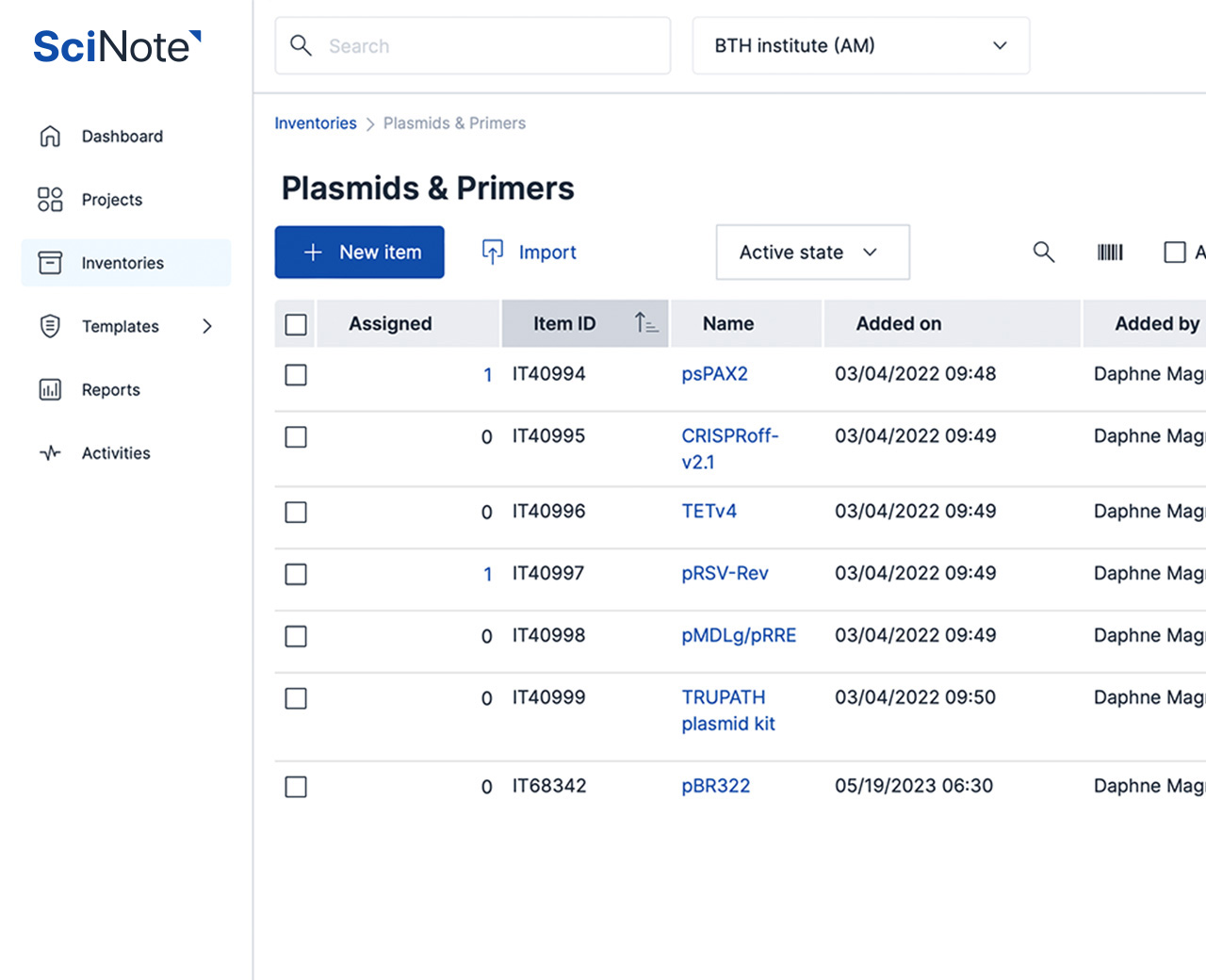
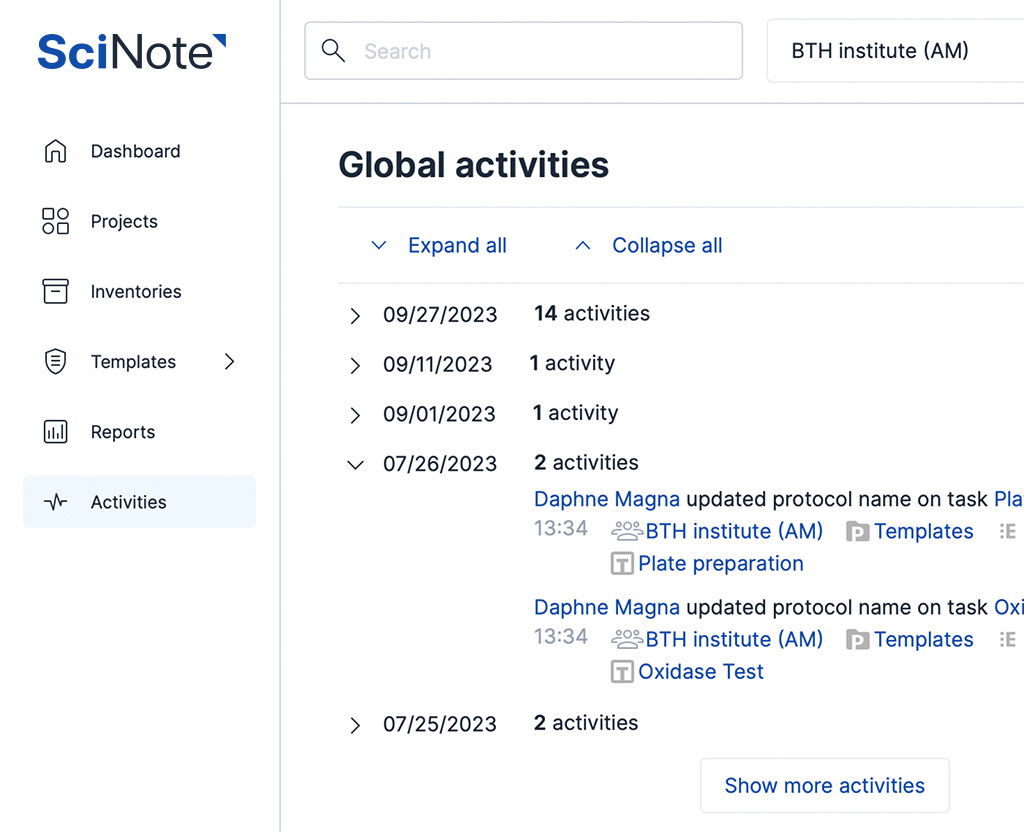
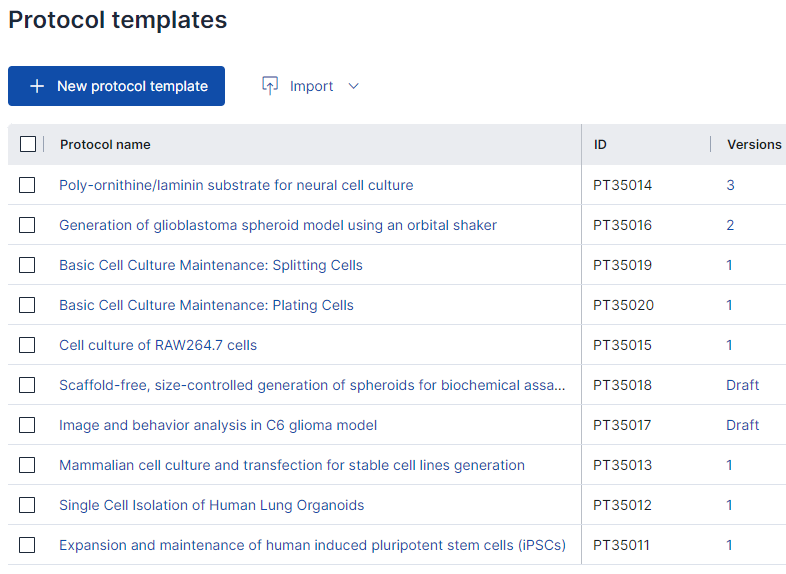
Manage your samples along with clinical, pathological, diagnostic, research and other data in a single platform. Connect biobanking capabilities for sample management with a powerful inventory system, experiment document systems, SOP library and robust cross-team collaboration features underlined with a GxP-grade traceability.
SciNote is ISO 27001:2022 certified and SOC 2 compliant, trusted by the FDA, USDA, and leading institutions driving clinical research and drug development:
Explore how SciNote can improve your
What makes SciNote stand out

“Gilson is proud to collaborate with SciNote, a top-rated lab digitalization software for industry researchers and their teams.
The connection between Gilson devices and SciNote helps scientists tackle the reproducibility issues that may arise. Keeping all your records in one place helps you know exactly how your experiments were conducted, connect your findings, raw data, reports and much more.”