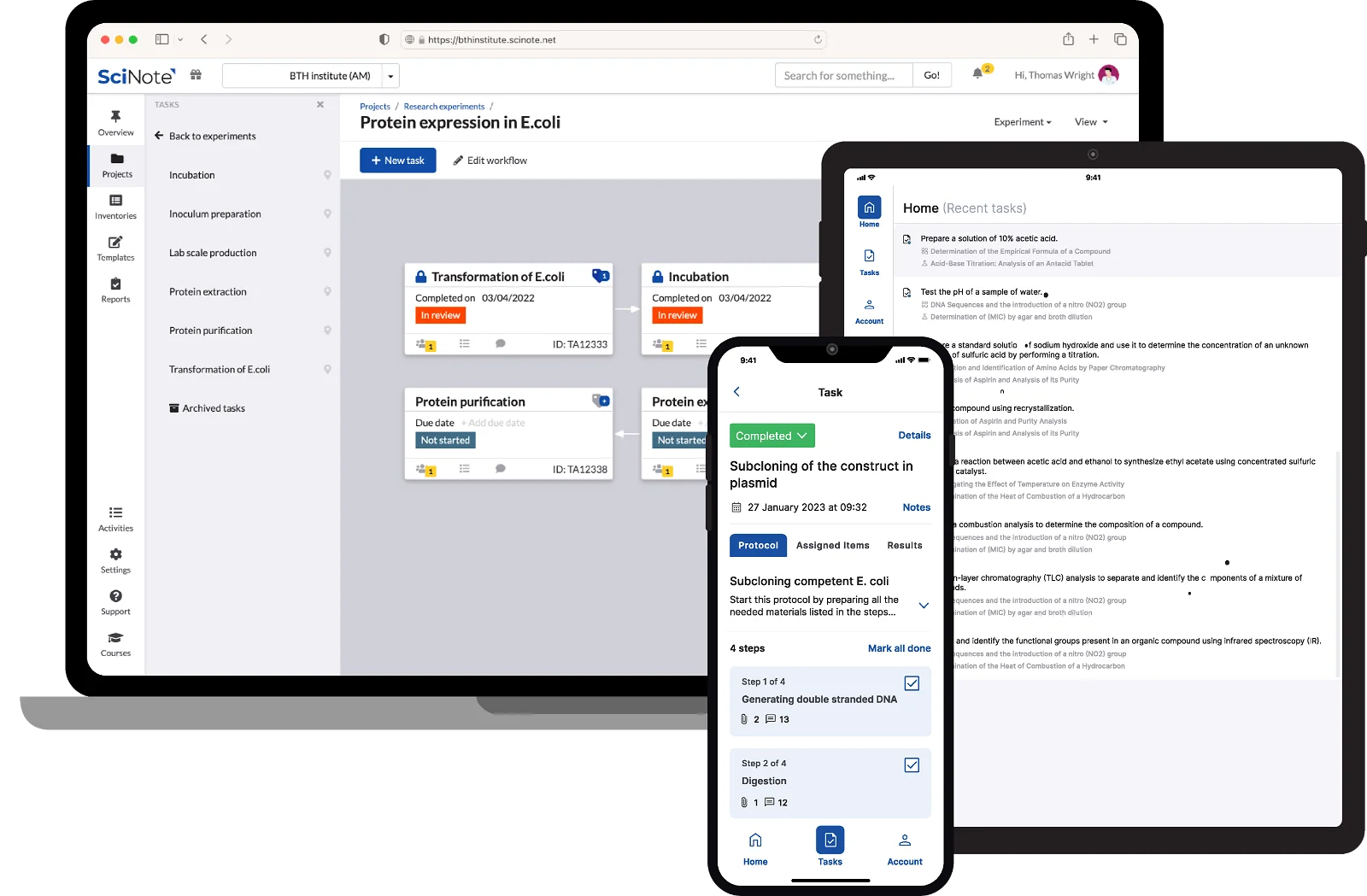
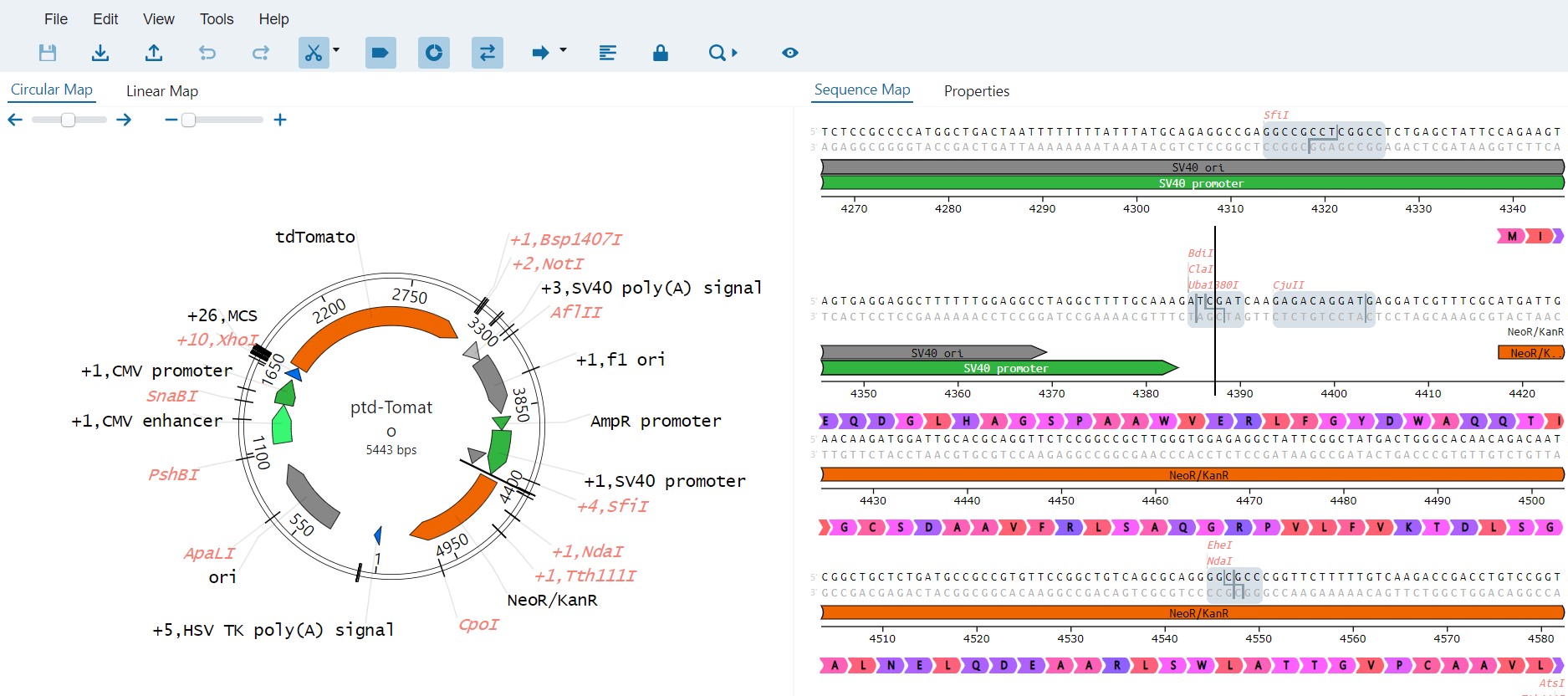
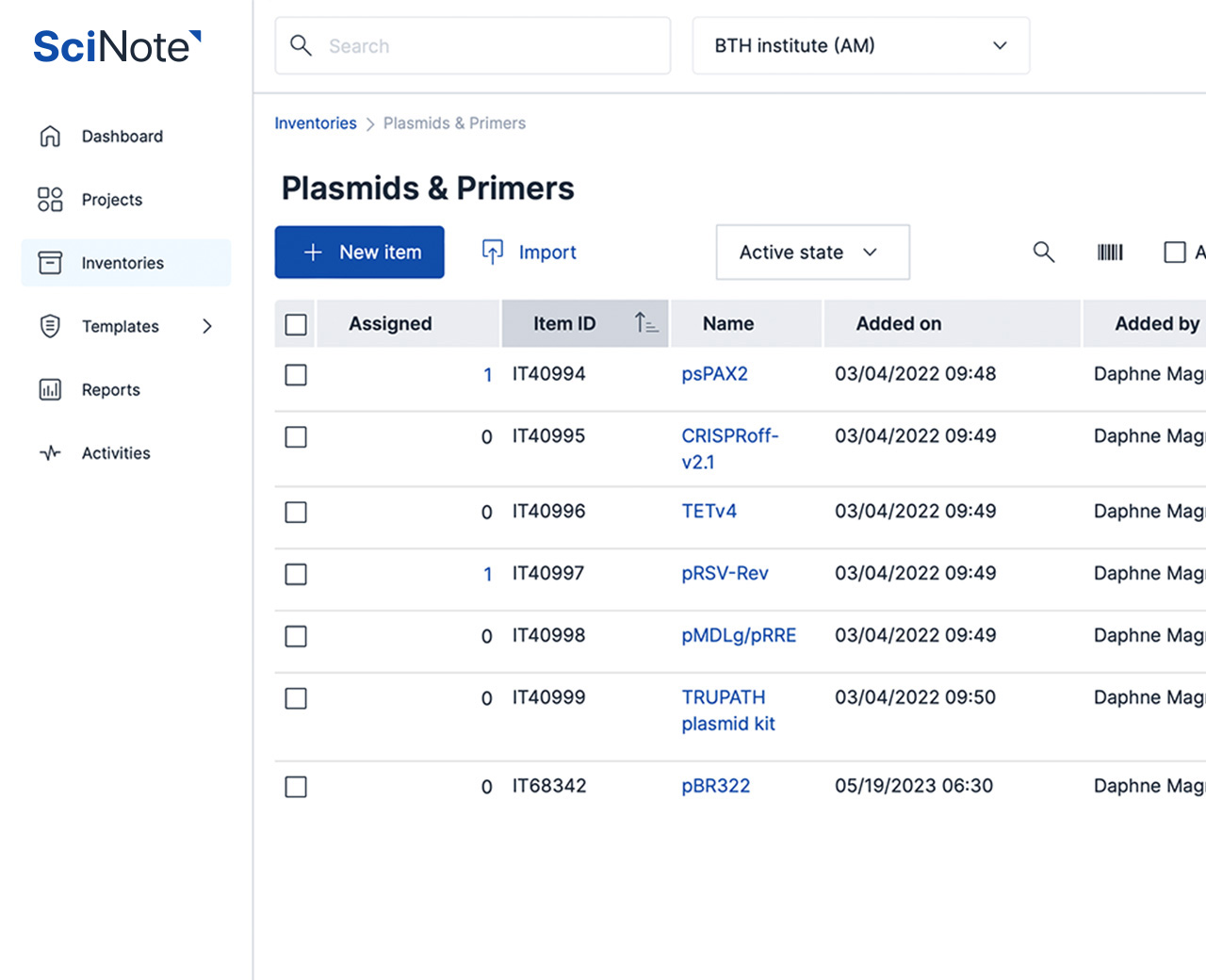
SciNote is currently in the FedRAMP Authorization process as a part of our commitment to customer needs and the trust they place in our product. The Federal Risk and Authorization Management Program (FedRAMP®) is a United States federal government-wide compliance program that provides a standardized approach to security assessment, authorization, and continuous monitoring for cloud products and services. It provides a standardized approach to security authorizations for Cloud Service Offerings.
Our security standards align with and go beyond the requirements of many security certifications. Upon request, we will provide supporting documents to demonstrate our information security management system (ISMS), as defined by ISO/IEC 27001.
SciNote is used at the FDA, USDA and other industry organizations. Learn more about the steps we take toward data protection in our Data Protection Whitepaper.
If you have any additional questions regarding our data protection and certification progress, please contact us at premium@scinote.net.